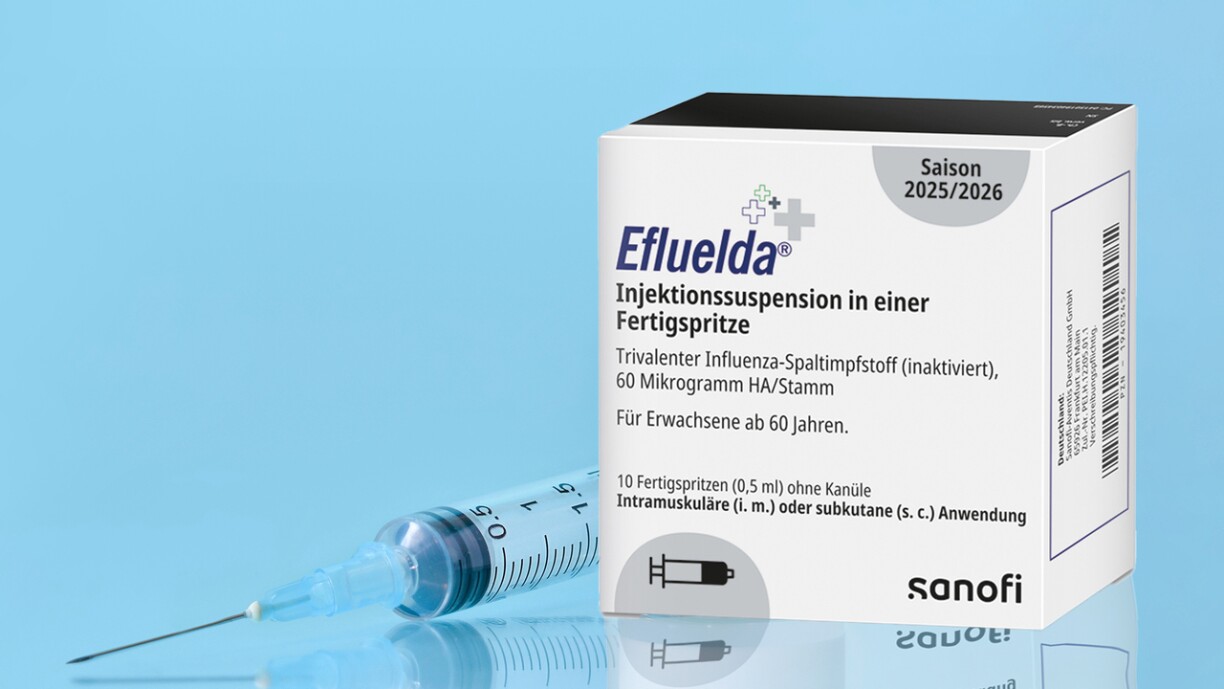
The National Health Directorate is recommending a new, high-dose flu vaccine for individuals over 65 ahead of this year’s vaccination campaign. However, a significant supply issue has emerged, as pharmacists report the recommended vaccine is not yet available in the country.
The annual autumn vaccination drive, officially starting on 15 October, primarily targets older adults, pregnant individuals, and those with chronic conditions such as heart or lung disease, obesity, and diabetes.
The recommendation for the new vaccine, named Efluelda, was communicated to doctors and pharmacists in a joint circular from the Health Directorate and the National Health Fund (CNS) on 22 September. According to Alain de Bourcy, president of the pharmacists’ association, the high-dose formulation is designed to create a stronger immune response in older adults, whose immune systems may not react sufficiently to standard vaccines. He noted that the stronger dose is not recommended for younger populations, who do not require it and may experience more pronounced side effects.
The central problem is one of availability. As Efluelda is new to the Luxembourg market, pharmaceutical wholesalers do not currently have it in stock. While an order has been placed, pharmacists do not expect the vaccine to arrive in pharmacies until the second week of October at the earliest.
This supply bottleneck is causing disruptions, unsettling patients, and forcing general practitioners to potentially reschedule vaccination appointments. Additionally, pharmacists risk being left with excess stock of the conventional flu vaccine they ordered based on previous guidelines.
De Bourcy criticised the timing of the new recommendation, stating that pharmacists typically place their vaccine orders in March and confirm them during the summer. The association contends that the National Health Directorate acted too late by convening the High Council of Infectious Diseases at an insufficiently early date to evaluate the new vaccine.
Expressing his frustration on behalf of the profession, de Bourcy described the situation as “sheer negligence” and stated that officials had been “asleep at the wheel.” He clarified that his criticism was not directed at Health Minister Martine Deprez personally, but rather at the management of the National Health Directorate. “These are the people who, technocratically and technically, have to ensure that this works – and nothing has happened,” he said.
De Bourcy pointed to more advanced planning abroad, citing Germany as an example where the Standing Vaccination Committee (STIKO) had already approved the Efluelda vaccine in October 2024 for the following season.
When contacted for a response, Dr Jean-Claude Schmit, Director of the National Health Directorate, declined to address the specific accusations directly, noting that Minister Deprez must first answer a formal parliamentary question on the matter. In an interview with our colleagues from RTL Télé, he commented generally on vaccine availability, stating, “The conventional vaccines are already here. The new vaccine, which is now an additional option, will arrive in the next few days. So there should be enough vaccines available for everyone.”
Approximately 80,000 to 90,000 people receive a flu vaccination in Luxembourg each year – a rate the health authorities have stated they are not fully satisfied with.